KU Leuven has developed a greener and safer alternative method for the dissolution of gold and platinum group metals. Strongly oxidizing conditions are required for the dissolution of noble metals. The designed leaching system requires mild conditions and was tested both on pure metal wires and spent automotive catalysts.
By using highly concentrated solutions of AlCl3·6H2O and Al(NO3)3·9H2O it was possible to leach 95% palladium from spent automotive catalysts in just 15 min at 80 °C. The dissolution of platinum and rhodium required longer times. Quantitative dissolution of palladium from the catalysts powder was achieved in a short time (15 min); about 64% platinum can be dissolved when the contact time was increased up to 4h.
The recovery of palladium was investigated by reductive precipitation with ascorbic acid however, but further investigation is required in order to optimize the PGM recovery. The principle behind this process is that concentrated solutions of salts of highly valent metal ions, having an H2O: metal-ion ratio sufficiently low to minimize outer-sphere hydration, show a marked degree of acidity which can be exploited for the dissolution of noble metals. The process represents a greener and safer alternative to the conventional aqua regia that is used to dissolve these types of metals.
Gallery 1. Palladium wire in contact with the molten salt hydrate mixture, after 3 min (a), 1 h (b), 2 h (c), 3 h (d), 4 h (e) and 4.5 h (f) leaching; S/C ratio = 35 g/g, T = 80 °C, water added = 22 wt%, stirring speed = 350 rpm.

a) 
b) 
c) 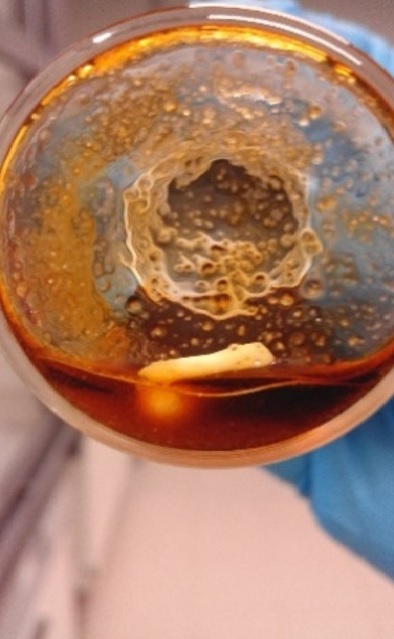
d) 
e) 
f)
This work has been published in the journal Chemical Communications and can be found here https://pubs.rsc.org/is/content/articlelanding/2020/cc/d0cc02298e#!divAbstract.
